Hello readers,
Here I will be exploring the science behind a potato cannon. However I recommend you to actually see the structure and building of a potato cannon before reading this in order to understand the terminology I will be using. So click this link if you wish to see a detailed guide on how I built a potato cannon and my tips and tricks if you plan on building one yourself. 👇
The combustion chamber-
The combustion chamber is the space where the igniter is actually placed and where the hairspray is present. When the igniter is lit up in the presence of propane in a space with a fixed volume an interesting thing is observed. Boyle’s Law tells us that the volume of gas increases as the pressure decreases. Charles’ Law tells us that the volume of gas increases as the temperature increases.
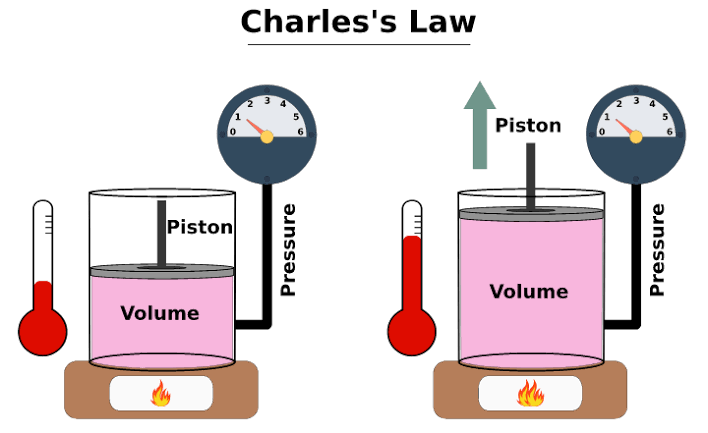
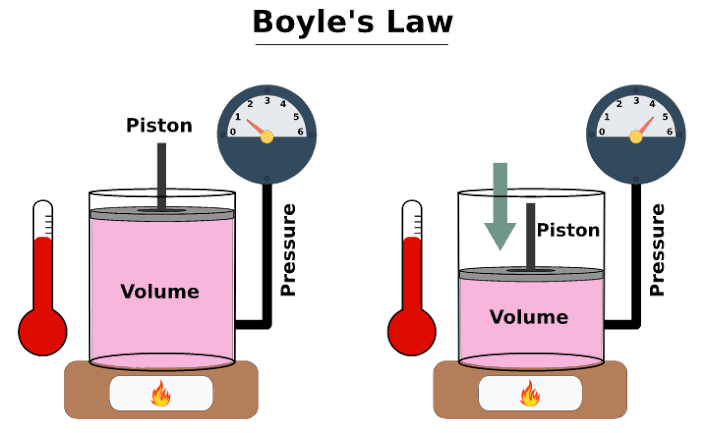
This tells us that if volume of a container is kept constant and the temperature of the gas in the container is increased, the pressure inside the container increase. This is exactly what is happening in this combustion chamber. The propane ignites and as a result the temperature of the gas increases. As the volume of the container is constant, the increase in temperature results in a increase of pressure of gasses inside the combustion chamber, which exerts greater force on the walls of the container and the potato that is lodged into the 2” pipe.
The high pressure gas looks to flow from an area of high pressure in the chamber to an area of low pressure outside the chamber. The walls of the container are fixed in place and cannot move, however, the potato is placed in the launching tube in such a way that it is free to move and completely occupies the cross section area of the 2” pipe. This allows the potato to be launched out of the way of the expanding gasses since the gasses cannot escape around the potato and have to instead push the potato out to flow from an area of high pressure to an area of low pressure.
Other interesting phenomena-
-You may observe water residue in the combustion chamber as a result of the reaction between the propane and oxygen in the air. This can be expressed by the formula-
C3H8 + 5O2 -> 3CO2 + 4H20 OR Propane + Oxygen -> Carbon Dioxide + Water
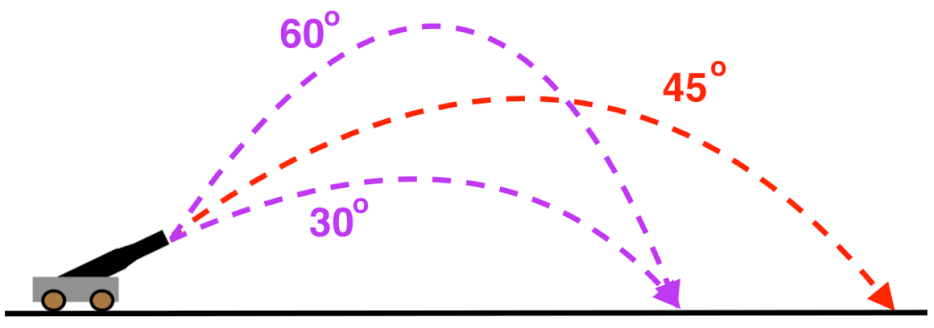
-To achieve maximum distance of the potato, aim the canon at 45 degree angle from ground. This can be demonstrated using this diagram. To achieve maximum power, use more hairspray.. however be carful to not go overboard (not more than 10 seconds) as it can result in the chamber exploding.